The principle of electrochemical energy storage has been known for over 200 years. The search for the perfect type of battery cells has taken almost as long. What is behind it, when is the big leap coming?
As is well known, the world does not always deal fairly with those who do important things. Johann Wilhelm Ritter would have seen it that way too, because the pharmacist and physicist, born in Silesia, died impoverished in Munich in 1810 at the age of 33. He built the first rechargeable battery, the so-called Ritter’s charge column, just a few years earlier by stacking copper disks and pieces of cardboard soaked in saline solution on top of each other in a glass tube. After the tube is charged, it will continue to supply power even if the charger is removed.
A state-of-the-art lithium-polymer battery works basically the same in a current electric car. A rechargeable battery stores electrical energy chemically and releases it again as electrical energy. With every conversion, part of the energy supplied is left behind, as the laws of thermodynamics want. The function of the batteries is based on the electronegativity of metals, more simply: on their property of releasing electrons.
Lithium comes into play
The atoms of lithium, the most electronegative of all metals and after hydrogen and helium the atomically lightest element in the periodic table are the most suitable. The lithium atoms particularly like to release one of their three electrons, which makes lithium a popular raw material for batteries. However, this property also means that the lithium atoms like to form chemical bonds with other atoms and molecules. So they have to be protected in the battery and kept away from air or water, which is associated with additional weight. Another disadvantage: Although lithium is by no means rare on earth, it is present in such a low concentration that economic promotion is only possible in a few places. Nevertheless, a large part of battery research is focused on lithium-ion batteries.
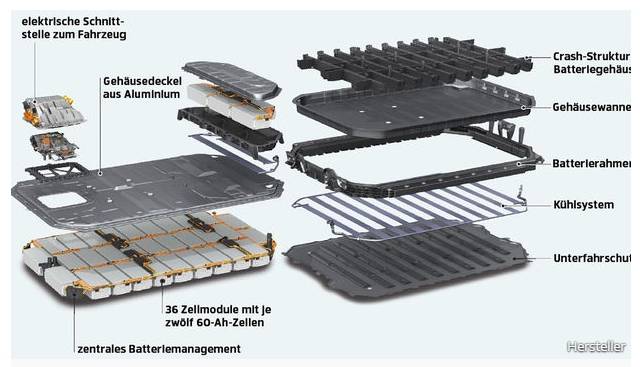
Put simply, lithium atoms give off their surplus electrons at the anode during discharge. The electrons flow through the conductor and consumer to the positive electrode, the cathode. The reverse process takes place during loading. The freshly charged lithium ions then transport electrons again through the electrolyte and separating layer to the anode. In reality, everything is a bit more complicated, but you definitely don’t want to read anything about carbon intercalation compounds here.
The first lithium-ion battery was researched and developed in Munich at the beginning of the 1970s, but there was no practical application. Only with the spread of energy-hungry consumer electronics and mobile telephony from the mid-1980s did the lithium-ion battery come into discussion. And in the electric car. A permanent problem of any battery technology is the energy density, lithium ion technology has changed a little, but not much. The energy density describes the relationship between mass and energy content of an energy source and is measured in megajoules per kilogram (MJ / kg).











More Stories
Tesla Introduces 84-Month Loans in the US Amid Rising Interest Rates
Opel automobiles: 125 years of history and a bright future
The Cars That Go Out of Production in 2023